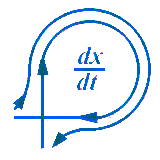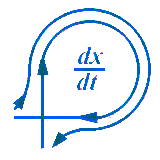Kinetic model of the ammonium chloride sublimation process
Author(s):
Maksim Igorevich Kuzmin
Researcher, Group of the Software Development, Modeling and Digitalization,
N.P. Sazhin State Research and Design Institute of Rare Metal Industry "Giredmet",
Postgraduate student, Department of Information Computer Technologies, D. Mendeleev University of
Chemical Technology of Russia
mimikatz@mail.ru
David Ilyich Kushniruk
Head of the group, Group of the Software Development, Modeling and Digitalization,
N.P. Sazhin State Research and Design Institute of Rare Metal Industry "Giredmet"
DIKushniruk@rosatom.ru
Nikita Sergeevich Romanov
Junior researcher, Department of film materials technology, S.E. Vyatkin Scientific Research Institute of
Graphite-based Structural Materials "NIIgrafit",
Postgraduate student, Department of Nanomaterials and Nanotechnology, D. Mendeleev University of
Chemical Technology of Russia
NSRomanov@rosatom.ru
Egor Andreevich Danilov
Head of the department, Department of Functional Materials, S.E. Vyatkin Scientific Research
Institute of Graphite-based Structural Materials "NIIgrafit"
EgADanilov@rosatom.ru
Anastasia Nikolaevna Babkina
Ph.D., Associate Professor, Department of Optoinformational Materials and Technologies, ITMO National Research University
babkina.anastasya@bk.ru
Abstract:
The article discusses the process of sublimation of chemically pure ammonium chloride crystals.
Instrumental studies were conducted using synchronous thermal analysis, including thermogravimetry and
differential scanning calorimetry, under non-isothermal conditions with three different heating rates (3, 5, and 10 В°C/min).
The data obtained from the experiments were processed using the Friedman isoconversion method.
Based on the assumption that the sublimation process consists of three successive stages that can
be modeled by n-th order reaction kinetics, a kinetic model was developed to describe the change in
sample mass over time. Statistical and nonlinear regression techniques were used to determine the kinetic
triplets for each stage. The model was then applied to predict the progression of the sublimation process
under various temperature regimes. The results of the work can be used both in an independent form and in the
construction of complex models of chemical processes in which it is necessary to take into account the course of a
competing process of sublimation of ammonium chloride.
Keywords
- ammonium chloride
- differential scanning calorimetry
- kinetic model
- sublimation
- thermogravimetric analysis
References:
- Grineva O., Dyachenko A., Kraidenko R. Chlorination of copper-containing raw material by ammonium chloride. Russian Journal of Applied Chemistry, 2013; 86:339-342
- Hö lsä J., Niinistö L. Thermoanalytical study on the reactions of selected rare earth oxides with ammonium halides. Thermochimica acta, 1980; 37(2):155-160
- Kim Y., Planinsek F., Beaudry B., Gschneidner K. Preparation and Purification of GdCl3. The Rare Earths in Modern Science and Technology, 1980; 2:53-58
- Meyer G., Ax P. An analysis of the ammonium chloride route to anhydrous rare-earth metal chlorides. Materials Research Bulletin, 1982; 17(11):1447-1455
- Meyer G., Garcia E., Corbett J. D. The ammonium chloride route to anhydrous rare earth chlorides—The example of YCl3. Inorganic Syntheses, 1989; 25:146-150
- Shibata H., Hayashi H., Minato K. Preparation of gadolinium chloride without using corrosive gases. Japan Atomic Energy Agency, 2007; 38(47):24
- Xing Z., Cheng G., Yang H., Xue X., Jiang P. Mechanism and application of the ore with chlorination treatment: A review. Minerals Engineering, 2020; 154:106404
- Xiong X., Li G., Pang Z., Chen S., Zou X., Xu Q., Cheng H., Li S., Zhu K., Lu X. Experimental and computational approaches to study the chlorination mechanism of pentlandite with ammonium chloride. RSC advances, 2022; 12(30):19232-19239
- Levy H. A., Peterson S. Neutron diffraction study of the crystal structure of ammonium chloride. Physical Review, 1952; 86(5):766
- Costich P. S., Maass Jr G. J., Smith N. O. Transitions in Ammonium Chloride-Ammonium Bromide Solid Solutions. Journal of Chemical and Engineering Data, 1963; 8(1):26-27
- Kennedy S., Patterson J., Chaplin R., Mackay A. The transformation of CsCl type ⇆ NaCl type. Part II: Orientation relations in phase transformations CsCl type → NaCl type in ammonium halides. Journal of Solid State Chemistry, 1974; 10(1):102-107
- Popov M., Galchenko G. Determination of the true heat capacity of powdered bodies at high temperatures. Zhurnal obshchej himii, 1951; 21(12):2220-2235. (In Russ. )
- Haynes W. M. CRC Handbook of Chemistry and Physics. Los Angeles, CRC Press, 2014. 2670 p
- Rassow H. Einfache Methode zur Bestimmungen von Schmelzpunkten und kritischen Temperaturen. Zeitschrift fü r anorganische und allgemeine Chemie, 1920; 114(1):117-150
- Chaiken R., Sibbett D., Sutherland J., Van de Mark D., Wheeler A. Rate of sublimation of ammonium halides. The Journal of Chemical Physics, 1962; 37(10):2311-2318
- Schultz R. D., Dekker A. O. The effect of physical adsorption on the absolute decomposition rates of crystalline ammonium chloride and cupric sulfate trihydrate. The Journal of Physical Chemistry, 1956; 60(8):1095-1100
- Spingler H. Kinetics of Vaporization of NH4Cl. Z. Physik. Chem. B, 1942; 52:90-116
- Knacke O., Stranski I., Wolff G. Zur Theorie der Verdampfungsgeschwindigkeit. Zeitschrift fü r Physikalische Chemie, 1951; 198(1):157-185
- Stephenson C. The dissociation of ammonium chloride. The Journal of Chemical Physics, 1944; 12(7):318-319
- Zhu R., Wang J. -H., Lin M. -C. Sublimation of ammonium salts: A mechanism revealed by a first-principles study of the NH4Cl system. The Journal of Physical Chemistry C, 2007; 111(37):13831-13838
- Vyazovkin S., Burnham A. K., Criado J. M., Pé rez-Maqueda L. A., Popescu C., Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochimica acta, 2011; 520(1-2):1-19
- Vyazovkin S., Burnham A. K., Favergeon L., Koga N., Moukhina E., Pé rez-Maqueda L. A., Sbirrazzuoli N. ICTAC Kinetics Committee recommendations for analysis of multi-step kinetics. Thermochimica acta, 2020; 689:178597
- Vyazovkin S., Chrissafis K., Di Lorenzo M. L., Koga N., Pijolat M., Roduit B., Sbirrazzuoli N., Suñ ol J. J. ICTAC Kinetics Committee recommendations for collecting experimental thermal analysis data for kinetic computations. Thermochimica acta, 2014; 590:1-23
- Deng C., Cai J., Liu R. Kinetic analysis of solid-state reactions: evaluation of approximations to temperature integral and their applications. Solid state sciences, 2009; 11(8):1375-1379
- Flynn J. H. The ‘temperature integral’—its use and abuse. Thermochimica acta, 1997; 300(1-2):83-92
- Orfao J. J. Review and evaluation of the approximations to the temperature integral. AIChE Journal, 2007; 53(11):2905-2915
- Friedman H. L. Kinetics of thermal degradation of char‐ forming plastics from thermogravimetry. Application to a phenolic plastic. Journal of polymer science part C: polymer symposia, 1964; 6:183-195
- Vyazovkin S. Modification of the integral isoconversional method to account for variation in the activation energy. Journal of Computational Chemistry, 2001; 22(2):178-183
- Vyazovkin S. Evaluation of activation energy of thermally stimulated solid‐ state reactions under arbitrary variation of temperature. Journal of computational chemistry, 1997; 18(3):393-402
- Ortega A. A simple and precise linear integral method for isoconversional data. Thermochimica acta, 2008; 474(1-2):81-86
- Vyazovkin S. Isoconversional kinetics of thermally stimulated processes. Switzerland, Springer, 2015. 239 p
- Savitzky A. A historic collaboration. Analytical Chemistry, 1989; 61(15):921A-923A
- Savitzky A., Golay M. J. Smoothing and differentiation of data by simplified least squares procedures. Analytical chemistry, 1964; 36(8):1627-1639
- Koga N., Goshi Y., Yamada S., Pé rez-Maqueda L. A. Kinetic approach to partially overlapped thermal decomposition processes: co-precipitated zinc carbonates. Journal of thermal analysis and calorimetry, 2013; 111:1463-1474
- Di Marco V. B., Bombi G. G. Mathematical functions for the representation of chromatographic peaks. Journal of Chromatography A, 2001; 931(1-2):1-30
- Fraser R. D., Suzuki E. Resolution of overlapping bands. Functions for simulating band shapes. Analytical chemistry, 1969; 41(1):37-39
- Fraser R. D., Suzuki E. Resolution of overlapping absorption bands by least squares procedures. Analytical Chemistry, 1966; 38(12):1770-1773
- Chen C., Miao W., Zhou C., Wu H. Thermogravimetric pyrolysis kinetics of bamboo waste via Asymmetric Double Sigmoidal (Asym2sig) function deconvolution. Bioresource technology, 2017; 225:48-57
- Svoboda R., Má lek J. Applicability of Fraser-Suzuki function in kinetic analysis of complex crystallization processes. Journal of thermal analysis and calorimetry, 2013; 111:1045-1056
- Yan Q. -L., Zeman S., Zhang J. -G., Qi X. -F., Li T., Musil T. s. Multistep thermolysis mechanisms of azido-s-triazine derivatives and kinetic compensation effects for the rate-limiting processes. The Journal of Physical Chemistry C, 2015; 119(27):14861-14872
- Khawam A., Flanagan D. R. Solid-state kinetic models: basics and mathematical fundamentals. The journal of physical chemistry B, 2006; 110(35):17315-17328
- Levenberg K. A method for the solution of certain non-linear problems in least squares. Quarterly of applied mathematics, 1944; 2(2):164-168
- Marquardt D. W. An algorithm for least-squares estimation of nonlinear parameters. Journal of the society for Industrial and Applied Mathematics, 1963; 11(2):431-441
- Opfermann J. Rechentechnik, Datenverarbeitung, 1985; 23:26
- Opfermann J. Kinetic analysis using multivariate non-linear regression. I. Basic concepts. Journal of thermal analysis and calorimetry, 2000; 60(2):641-658
- Dormand J. R., Prince P. J. A family of embedded Runge-Kutta formulae. Journal of computational and applied mathematics, 1980; 6(1):19-26